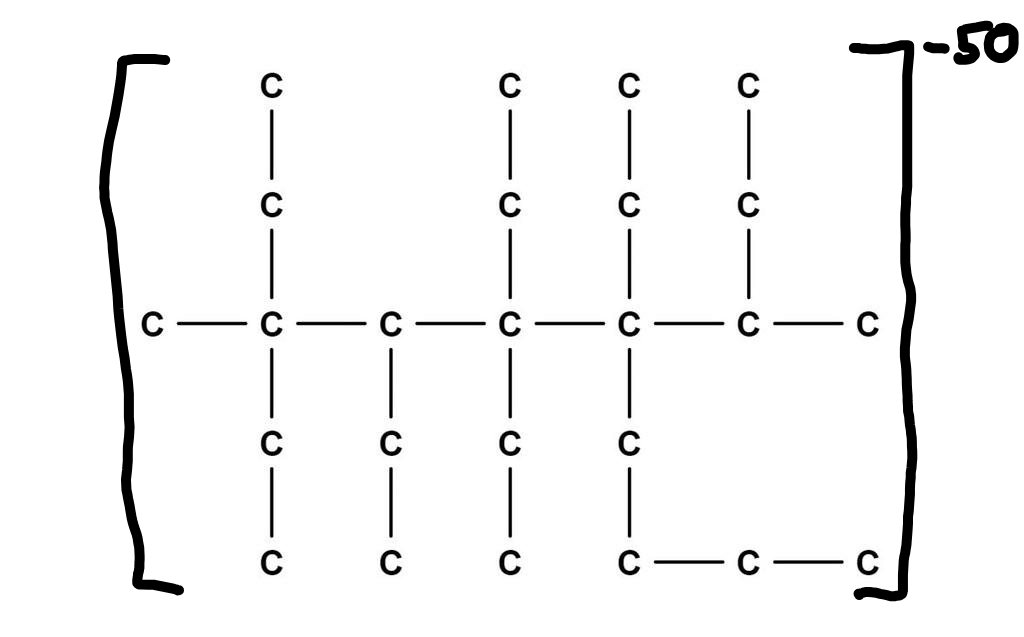
Fixed the charge on your 3-methyl-3,4,5,5,6-pentaethyl-6-butan-2-yl decane ion, aka lossane.
(It’s been a while since I last did chemistry, so apologies if I messed up the nomenclature a bit)
As someone who paid enough attention in highschool chemistry to get a B, and occasionally watches Nile(red/blue) and E&I videos… I know some of these words/symbols!
Any idea what a molecule like that would be useful for?
Fuel? Hydrocarbons like that are quite combustible. It could also be incorporated into a lipid or something.
Couldn’t resist. In a more standard form this molecule should (I believe—chemistry has been a long time ago) look like this:
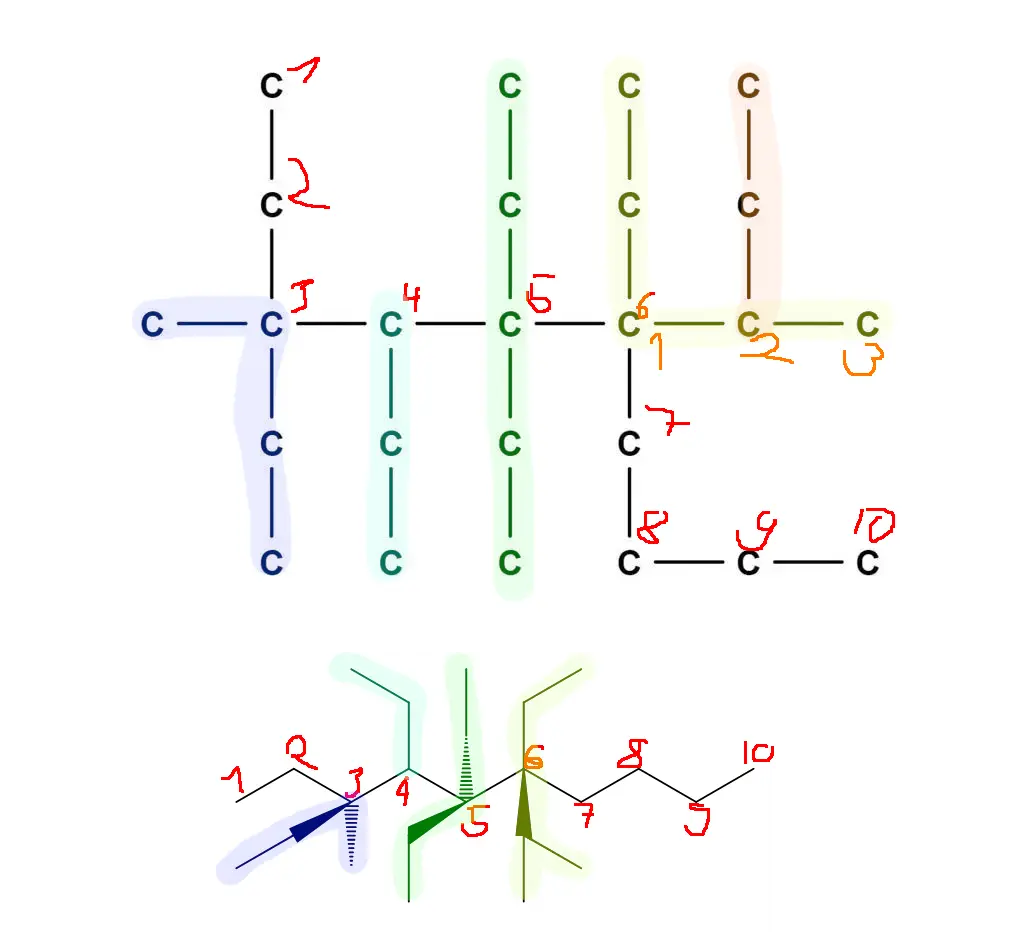
Made with MolView –https://molview.org/?smiles=C([C%40](CC)(C)C(CC)[C%40](CC)(CC)[C%40%40](C(C)C)(CC)CCCC)C
People knowledgeable in chemistry, please correct!
If I ever teach chemistry to kids imma tell them to name this
So … What does this chemical make you lose?
Sanity
Your marbles.
It makes you lose your way in a vast endless c
Assuming this has a 47 hydrogens stuck on to make it stable, I’d call it:
3-methyl-3,4,5,5,6-pentaethyl-6-buta-2-yl decane
dodecane has a 12 carbon chain. The longest chain here is 10 carbons, which would be decane.
Good catch.
I am a total chem nitwit, would you like to explain me how you come to this ?
It basically comes down to finding the longest chain of carbons, then you number each of the carbons on that chain and list off things that are attached to each of them. For example, 1 carbon = methyl, 2 carbons = ethyl, etc.
(1,2,2,50)-loss-quinquagintinane
The urge to add hydrogens to all the carbons not fully bonded is overwhelming
I somehow recognized it immediately. I think this meme has rewritten my brain.
My stoned ass thought this was a shifter tree diagram of a goofy little manual transmission for a sec. All gears are just ‘crash’, lol
…is this gain?
OK how long until it kicks in?
a few years and then you die of cancer
3-metilhexa-4,5,5,6,7,7-etil-4-butil From the head, im feel happy now