15
Background: The spike protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is crucial to viral entry and can cause cardiac injuries. Toll-like receptor 4 (TLR4) and NOD-, LPR-, and pyrin-domain-containing 3 (NLRP3) inflammasome are critical immune system components implicated in cardiac fibrosis. The spike protein activates NLRP3 inflammasome through TLR4 or angiotensin-converting enzyme 2 (ACE2) receptors, damaging various organs. However, the role of spike protein in cardiac fibrosis in humans, as well as its interactions with NLRP3 inflammasomes and TLR4, remain poorly understood. Methods: We utilized scratch assays, Western blotting, and immunofluorescence to evaluate the migration, fibrosis signaling, mitochondrial calcium levels, reactive oxygen species (ROS) production, and cell morphology of cultured human cardiac fibroblasts (CFs) treated with spike (S1) protein for 24 h with or without an anti-ACE2 neutralizing antibody, a TLR4 blocker, or an NLRP3 inhibitor. Results: S1 protein enhanced CFs migration and the expressions of collagen 1, α-smooth muscle actin, transforming growth factor β1 (TGF-β1), phosphorylated SMAD2/3, interleukin 1β (IL-1β), and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). S1 protein increased ROS production but did not affect mitochondrial calcium content and cell morphology. Treatment with an anti-ACE2 neutralizing antibody attenuated the effects of S1 protein on collagen 1 and TGF-β1 expressions. Moreover, NLRP3 (MCC950) and NF-kB inhibitors, but not the TLR4 inhibitor TAK-242, prevented the S1 protein-enhanced CFs migration and overexpression of collagen 1, TGF-β1, and IL-1β. Conclusion: S1 protein activates human CFs by priming NLRP3 inflammasomes through NF-κB signaling in an ACE2-dependent manner.


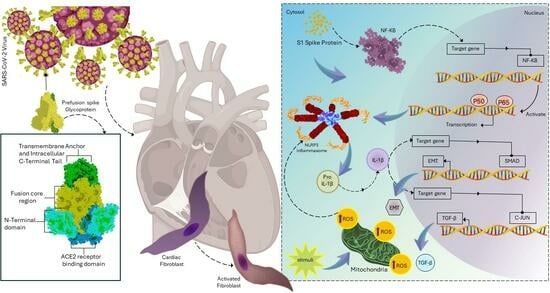
This is wild, TLR4 is associated with our immune system’s identification of LPS, which is found in the outer layer of gram-negative bacterial cells. So why it’s associated with the SARS-CoV-2 virus is mind-bending. For anyone interested in the TLR’s found in the immune system, here you go: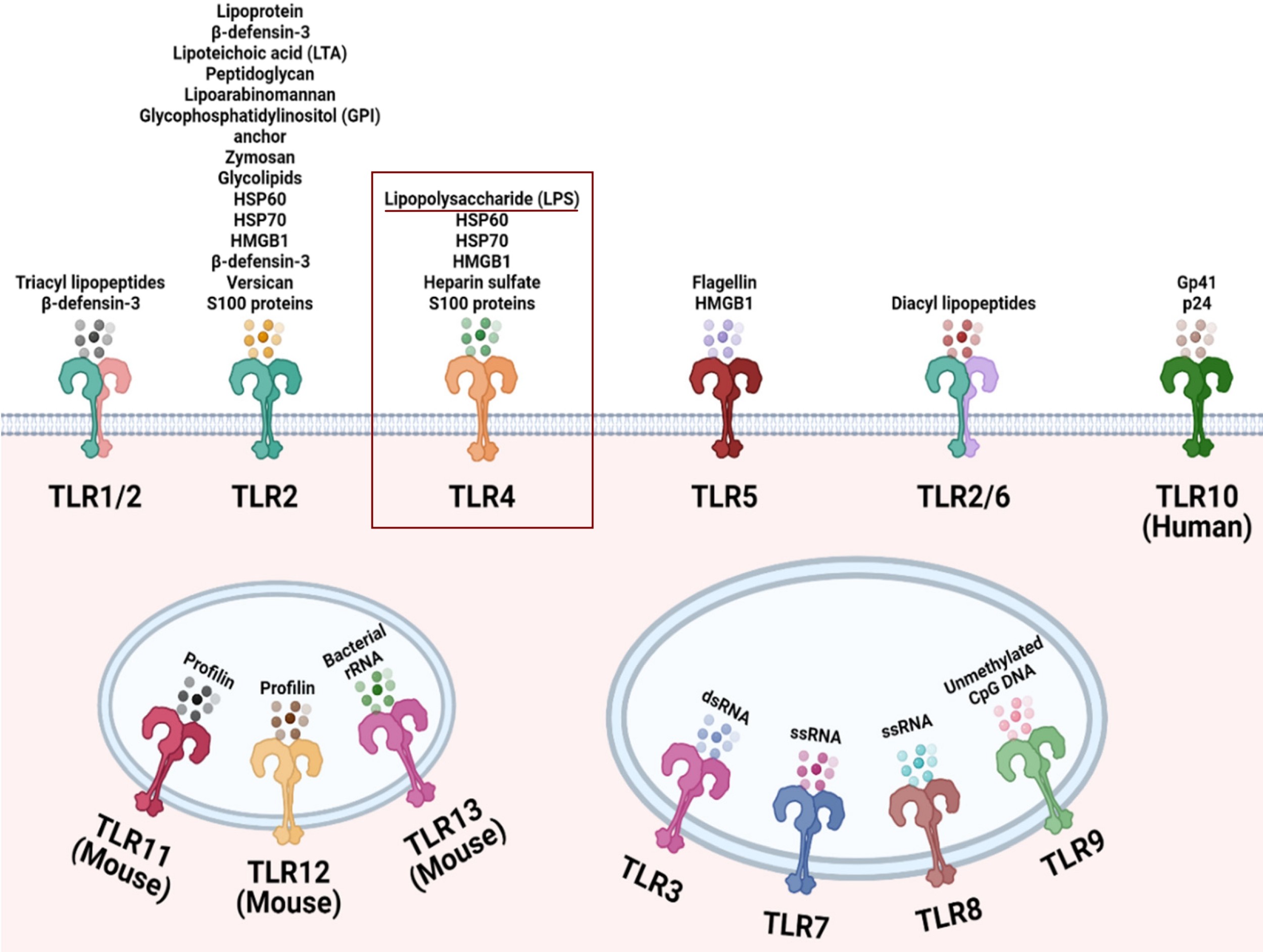
It’s well-known NF-kB is directly associated with NOD-Like-Receptors, which produces inflammation as an inflammasome. This is what allows immune cells to target the area in numbers. But the TLR4 involvement here is puzzling, would have 100% bet against its involvement in the immune response to SARS-CoV-2!
Edit: For clarity, S100 proteins are only found in vertebrates, and Heparan Sulfate is expressed by mammals on cell surfaces and in the surrounding extracellular matrix. HMGB1 works as a chromatin binding factor in our bodies. HSP60 + HSP70 (known as Heat Shock Proteins) are found in both prokaryotes and eukaryotes and are a response to stressful conditions. They’re basically chaperones and help control protein folding, transport, degradation, cell differentiation, and translocation. But viruses aren’t prokaryotes, so the HSPs they’re using during an infection are from the host. This helps show why I’m soo blown away with TLR4 being involved here!!